|
|
How Accurate is K-Ar Dating? |
|
|
|
How Accurate is K-Ar Dating? |
|
Laurence D Smart B.Sc.Agr., Dip.Ed., Grad.Dip.Ed
PO box 175, Kippax, ACT Australia 2615
Email: laurence@unmaskingevolution.com
Webpage: www.unmaskingevolution.com
[Free to print and distribute. Copy must be in full.]
Potassium-Argon Radiodating Theory
(1) The radioisotope 40K constitutes 0.012% of all naturally occurring potassium. S.T. Butler & H. Messel, "A Modern Introduction to Physics" (vol. 3), Howitz Pub. Inc. P/L & Grahame Book Co P/L: Sydney, p:24 1962
(2) 40K has a half-life of 1.31 billion years.
(3) 40K (parent isotope) breaks down to 40Ar (daughter isotope) by gaining an electron.
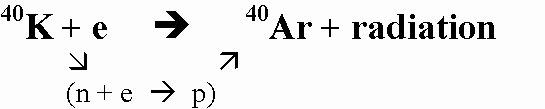
(4) "Because argon is a gas, the structures of minerals crystallizing from magma do not retain it, although many of these minerals may include rather abundant potassium. The radiogenic argon that builds up in potassium-rich minerals after they have crystallized, therefore, furnishes a good measure of the age of the sample." M.E. Bickford, et al (eds) "Geology Today", CRM Books: Del Mar (California), p:427 1973
(5) "One of the main uses of potassium-argon in recent years has been in determining the ages of quite young samples, for example, those less than 60 million years old. The rubidium-strontium and uranium-lead techniques are very difficult to use with such samples, because the slow decay rates of the parent isotopes have not allowed a significant increase in the daughter isotopes." M.E. Bickford, et al (eds) "Geology Today", CRM Books: Del Mar (California), p:427-428 1973
(6) "The principal difficulty with the potassium-argon method lies in the fact that argon is a gas and can escape from the crystals of minerals in which it accumulates. Commonly the ages of minerals from rather old rocks dated by the potassium-argon method are lower than the ages obtained by either the rubidium-strontium and uranium-lead dating. Moreover, many studies have demonstrated that argon escapes readily during metamorphic events when rocks become heated and partially crystallized." M.E. Bickford, et al (eds) "Geology Today", CRM Books: Del Mar (California), p:427 1973
Background to the K-Ar Dating Experiment
For example:
|
SITE |
ROCK TYPE |
DATE OF FORMATION |
K-Ar ASSESSED AGE |
|
Haualalai (Hawaii) |
Basalt |
AD 1800-1801 |
1.6 million years old |
|
Mt Etna (Sicily) |
Basalt |
122 BC |
0.25 million years old |
|
Mt Etna (Sicily) |
Basalt |
AD 1792 |
0.35 million years old |
|
Mt Lassen (California) |
Plagioclase |
AD 1915 |
0.11 million years old |
|
Sunset Crater (Arizona) |
Basalt |
AD 1064-1065 |
0.27 million years old |
[G.B. Dalrymple, Earth and Planetary Sciences Letters, Vol. 6, p:47-55 1969]
The Experiment
The Sample of Rock
(1) A 7 Kg sample of dacite was collected from the north-west slope of the lava dome formed from the 1986 flow.
(2) A 1 Kg block of rock was sawn from inside the sample. This sample had not been exposed to the argon in the air over the 10 years since it was formed.
(3) The chemical analysis of the sample showed that it was typical porphyritic dacite.
The Preparation of the Rock for Testing
(1) The test block was washed thoroughly to remove any outside contamination.
(2) The rock was crushed and milled using an iron mortar.
(3) Rock powder was sieved through thoroughly washed screens.
(4) The sieved material was washed to remove any contamination from the air.
(5) The resultant powder had a particle size of 0.180-0.075 mm.
(6) The powdered rock was filtered using heavy liquids to remove any contamination.
(7) Part of the powder was separated into four different mineral samples - feldspar-glass, heavy magnetic, heavy non-magnetic, and pyroxene.
(8) Each sample was scanned under the microscope to ensure that there were no foreign particles in them.
(9) The samples were stored in vials away from the air and dust.
The K-Ar Dating Test
(1) The 5 samples were analysed by the Geochron Laboratories, Cambridge, Massachusetts under the direction of Richard Reesman.
(2) The lab was not told where the rock came from, or that the age of the rock was known.
(3) Flame photometry was used to measure the amount of K (%).
(4) The amount of 40K (ppm) was calculated from the terrestrial isotopic abundance using the K concentration.
(5) The concentration of 40Ar ('radiogenic argon-40') was derived using a mass spectrometer.
(6) Two measurements for each element were taken and an average calculated from them.
(7) The ratio of 40Ar to the total Argon was measured using a mass spectrometer.
The Age Calculation
(1) The age was calculated using the 'general model-age equation'.
t=1/l ln [((Dt-Do)/Pt)+1]
t = the age of the rock
l = the decay constant for 40K (5.543x10-10/yr)
Dt = the number of 40Ar atoms in the rock when it was analysed
Do = the number of 40Ar atoms in the rock when it was formed
Pt = the number of 40K atoms in the rock when it was analysed (0.105)
(2) The equation becomes:- t=1/(5.543x10-10) ln [((1/0.105)(40Ar/40K))+1]
The Results
|
Mineral Sample |
40K [ppm] |
40Ar [ppm] |
Calculated Age [million years] |
|
Whole rock |
1.102 |
0.0000225 |
0.35 ± 0.05 |
|
Feldspar-glass |
1.250 |
0.0000250 |
0.34 ± 0.06 |
|
Heavy magnetic |
0.693 |
0.0000370 |
0.90 ± 0.20 |
|
Heavy non-magnetic |
0.555 |
0.0000540 |
1.70 ± 0.30 |
|
Pyroxene |
0.533 |
0.0000870 |
2.80 ± 0.60 |
Analysis of the Dating Method
(1) It was an assumption that: Dt-Do = 40Ar
(2) "As a matter of practice, no radiogenic argon is supposed to have existed when the rock was formed." CEN Tech. J., Vol. 10, No. 3, p:340 1996
(3) Because the rock was ten years old, there was no time for 40Ar to form from 40K. Therefore any 40Ar measured was not radiogenic argon.
(4) "The argon analysis of the dacite lava dome show, surprisingly, a non-zero concentration of 'radiogenic argon' (40Ar) in all preparations from the dacite." CEN Tech. J., Vol. 10, No. 3, p:340 1996
(5) Different minerals were shown to contain varying amounts of argon gas.
(6) Verifying this:-"The solubility of Ar in the minerals [olivine] is surprisingly high." Broadhurst, et al, Geochimica et Cosmochimica, Vol. 54, p:299-309
The Conclusion of the Test
Doesn't the Isochron Method Prove that K-Ar is Correct?
This research is supported by more Recent K-Ar Tests
The Implications of the Test
(A) The varying mineral composition of the myriads of types of rocks negates the accuracy of the standard potassium-argon dating method.
(B) The potassium-argon method should not be used to calculate the ages of rocks.
(C) As all new radiometric dating methods are calibrated using dates from existing methods, any based directly or indirectly on the potassium-argon method should not be used to calculate the ages of rocks.
In added support - "[We] should therefore not be intimidated by claims that U-Th-Pb radiometric 'dating' has 'proved' the presumed great antiquity of the earth, and the strata and fossils of the so-called geologic column." A.A. Snelling, "U-Th-Pb 'Dating': An example of False 'Isochrons' ", Proceedings of the Third International Conference on Creationism, Technical Symposium Sessions, 1994 p:503
SOURCE - CEN Tech. J., Vol. 10, No. 3, p:335-343 1996
